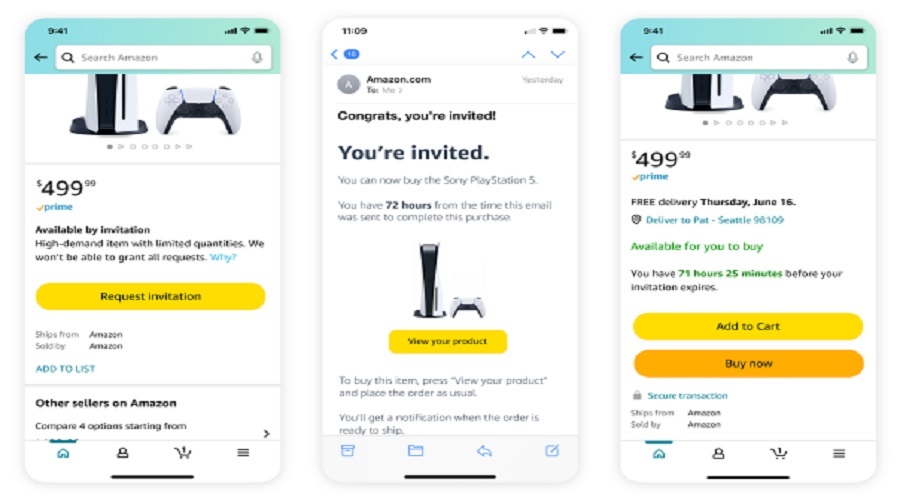
A Ban Imposed by FDA on Antibacterial Soaps Using Specific Chemicals
The U.S FDA (Food and Drug Administration) has announced in the month of September that the manufacturers of OTC (over-the-counter) consumer antiseptic was products having more than 19 specific active elements such as triclocarban and triclosan will not be permitted to sell their products. The FDA issued a statement that companies manufacturing those products, but […]